To understand Intrinsic Semiconductor Definition you must know about the types of materials and semiconductors.
Table of Contents
Materials are divided into conductors, semiconductors, and insulators. Semiconductors are the basic parts of all smartphones and computers. They are driving advancements in technology.
Semiconductor or Intrinsic Semiconductor Definition
A semiconductor is a material whose conduction lies between conductors and insulators, allowing them to control the flow of electrical current.
At zero Kelvin the semiconductors behaves as insulators and with increasing the temperature, they behaves as conductors. This special property makes semiconductors essential components in electronic devices, transistors, diodes, integrated circuits etc.
Applications of Semiconductors
Semiconductors have many applications in modern technology
- Used in Computers and Laptops
- Smartphones and Tablets
- Solar Panels
- LEDs and Displays
- Automotive Industry
- Medical Devices
- Consumer Electronics
- Telecommunications
- Industrial Automation
- Internet of Things (IoT)
Semiconductor Elements
Silicon (Si) and Germanium (Ge) are the mostly used semiconductors. Below are the few semiconductor elements.
| Semiconductor Material | Number of Electrons | Applications |
|---|---|---|
| Silicon (Si) | 14 | Microelectronics |
| Germanium (Ge) | 32 | Early transistors |
| Gallium Arsenide (GaAs) | 31 (Ga), 33 (As) | High-speed devices, optoelectronics |
| Silicon Carbide (SiC) | 14 (Si), 6 (C) | used in high-temperature and high-voltage power electronics |
| Gallium Nitride (GaN) | 31 (Ga), 7 (N) | High-efficiency LEDs, power amplifiers, RF components |
| Cadmium Telluride (CdTe) | 48 (Cd), 52 (Te) | Thin-film solar cells |
| Indium Phosphide (InP) | 49 (In), 15 (P) | High-frequency and high-power electronics, fiber optic communication systems |
| Organic Semiconductors | Varies (e.g., Carbon: 6) | Flexible electronics, organic LEDs (OLEDs), organic solar cells |
| Copper Indium Gallium Selenide (CIGS) | 29 (Cu), 49 (In), 31 (Ga), 34 (Se) | Thin-film solar cells for high-efficiency photovoltaic applications |
| Zinc Oxide (ZnO) | 30 (Zn), 8 (O) | Transparent electronics, sensors, UV light-emitting devices |
Types of semiconductors
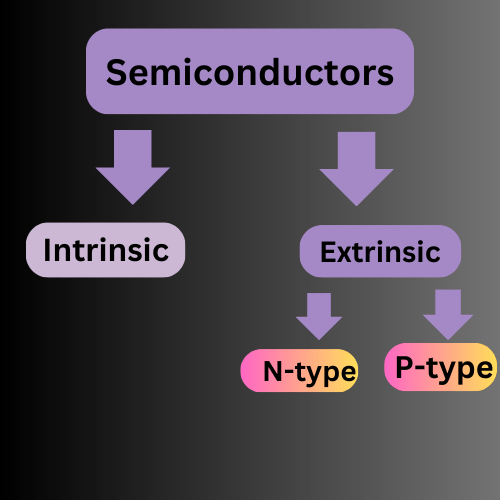
Semiconductors are divided into two types
- Intrinsic Semiconductors
- Extrinsic Semiconductors
Intrinsic Semiconductor Definition
Intrinsic Semiconductor is pure semiconductor without adding any impurity to them.
Examples are Silicon (Si), Germanium(Ge), and Gallium Arsenide (GaAs).
Extrinsic Semiconductor Definition
The semiconductor formed due to doping with impurity is called Extrinsic Semiconductor.
Doping is adding impurities to the intrinsic semiconductor to change their electrical properties.
There are two types of Extrinsic semiconductors.
- N-Type
- P-Type
How is N-type Formed?
N-type semiconductors are formed when intrinsic semiconductors(pure) are Doped with Pentavalent impurities.
Examples of pentavalent elements are Phosphorus(P), Arsenic (As), Antimony (Sb), and Bismuth (Bi).These elements have 5 valency electrons.
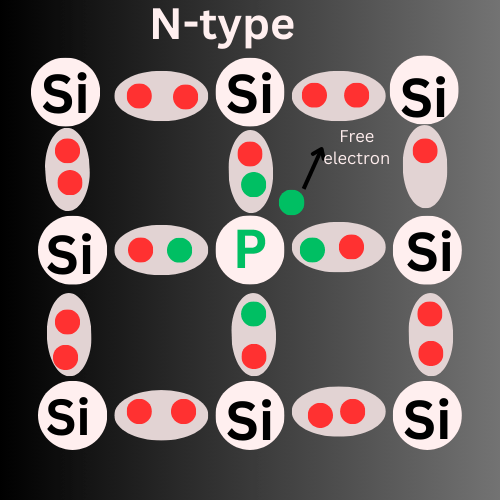
When we are adding phosphorus (five valency electrons) atom to silicon atom (four valency electrons), phosphorus atom donates one extra electron to the silicon. These extra electrons are called free electrons and the impurities are called donors. Here, Electrons are the majority charge carriers and holes are the minority carriers. The Free electrons are the reason for the electric current in N-type Semiconductors.
These free electrons act as negative charge carriers.
How is P-type Formed?
P-type semiconductors are formed when intrinsic semiconductors(pure) are Doped with Trivalent impurities.
Examples of trivalent elements are Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), and Thallium (Tl). These elements have three valency electrons.
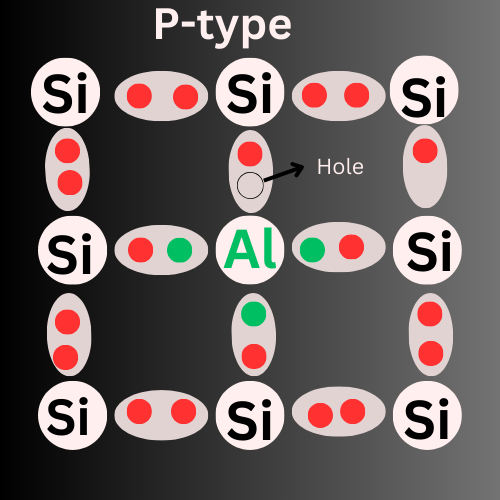
When Aluminum (three valency electrons) is added to silicon material(four valency electrons), Aluminum forms three covalent bonds with three valency electrons of Silicon(Si), where there is no electron to form a fourth covalent bond resulting in an empty space. The space created is called a hole and the impurities are called as acceptors. Here, holes are the majority charge carriers and electrons are the minority charge carriers in the P-type.
Hence, the holes are the reason for the electric current in P-type Semiconductors.
These holes act as positive charge carriers.
Comparison of p-type and n-type semiconductors
| Feature | P-Type | N-Type |
|---|---|---|
| Doping impurity | Trivalent (Ex: Boron, Aluminum, Gallium) | Pentavalent (Ex: Phosphorus, Arsenic) |
| Base substance | Pure semiconductor Ex: Silicon, Germanium | Pure semiconductor Ex: Silicon, Germanium |
| Majority Charge Carriers | Holes | Electrons |
| Minority Charge Carriers | Electrons | Holes |
| Conductivity | due to holes | due to free electrons |
| Energy Levels | Acceptors create holes near the valence band | Donors create extra electrons near the conduction band |
| Examples | Boron-doped Silicon (Si-B) | Phosphorus-doped Silicon (Si-P) |
| Gallium-doped Germanium (Ge-Ga) | Arsenic-doped Germanium (Ge-As) |
This above information gives the basic semiconductor definition, applications, Intrinsic Semiconductor Definition, Extrinsic Semiconductor Definition and its types.
